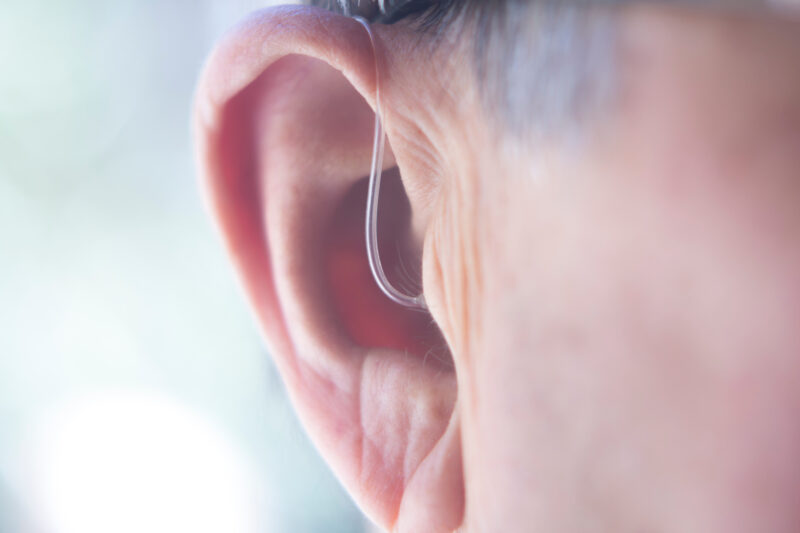
On October 19, 2021 health officials announced changes in regulations that would enable Americans to purchase over-the-counter (OTC) hearing aids without prescriptions or previously required hearing exams. This accessibility could also allow seniors and adults with impaired hearing to skip the pharmacy altogether and purchase their devices online.
This long-awaited proposal also means more accessible pricing for the 15% of Americans currently experiencing hearing difficulties.
While the FDA has not yet finalized the proposal, they are accepting public feedback for 90 days ahead of settlement on the new plan, allowing for eligible devices to be fully vetted before hitting the shelves. According to officials, OTC hearing aids will have stricter requirements to be met, however device approval will be determined by application rather than required consumer studies.

When will OTC hearing aids be available in stores?
The push to make OTC hearing aids dates back as far as 2018 when the FDA stated that they cannot label their products as OTC until final FDA regulations are published. The original deadline to release regulations was on August 18, 2020 but was delayed due to COVID-19. Assuming all goes according to plan, individuals can potentially see devices available for purchase in stores and online in early 2022.